FDA launches Agricultural Water Assessment Builder to help farms understand Agricultural Water Proposed Rule requirements | CDFA Inspection Services Blog

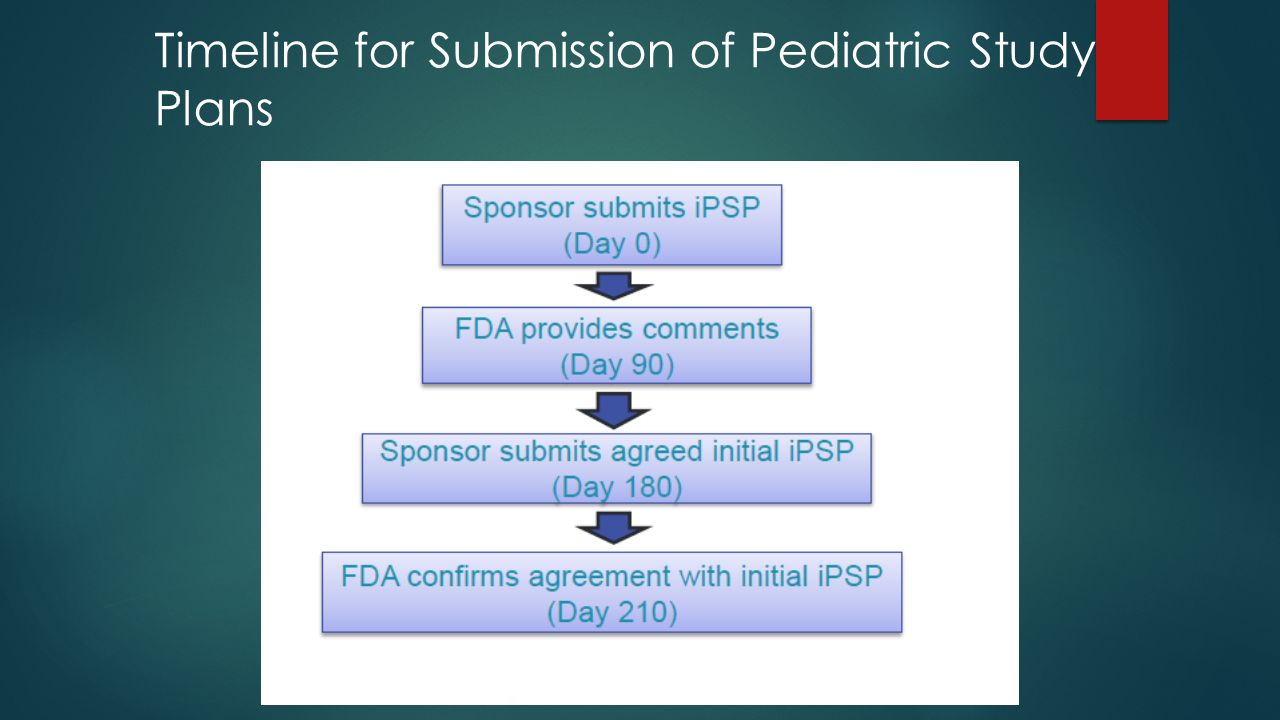
Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram

Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram

Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram

Paediatric drug regulatory process in EU and U.S. (source: FDA and EMA... | Download Scientific Diagram

Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram
FDA / EMA Common Commentary on Submitting an initial Pediatric Study Plan (iPSP) and Paediatric Investigation Plan (PIP) for the


![PDF] Pediatric drug development in Japan: Current issues and perspectives | Semantic Scholar PDF] Pediatric drug development in Japan: Current issues and perspectives | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f345b6914fe7338feb66ba7ebcb4f0498f088d32/5-Table2-1.png)