Teva launches first DTC for Austedo into competitive tardive dyskinesia 2-drug market | Fierce Pharma

Metabolic pathways of tetrabenazine and deutetrabenazine. RR enantiomer... | Download Scientific Diagram

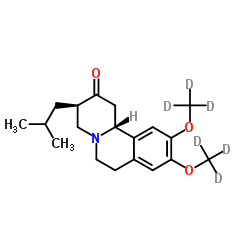
Pharmacokinetic and Metabolic Profile of Deutetrabenazine (TEV‐50717) Compared With Tetrabenazine in Healthy Volunteers - Schneider - 2020 - Clinical and Translational Science - Wiley Online Library
Teva Announces FDA Approval of AUSTEDO™ (deutetrabenazine) Tablets for the Treatment of Chorea Associated with Huntington's Disease - Chemdiv
Teva Announces FDA Acceptance of Resubmitted New Drug Application for SD-809 for Treatment of Chorea Associated with Huntington Disease - Chemdiv

Teva announces FDA approval of Austedo tablets for chorea associated with huntington's disease - Pharma Advancement

Teva Launches 'It's Not OK - It's TD' National Television Advertising Campaign to Increase Awareness of Tardive Dyskinesia and AUSTEDO® ( deutetrabenazine) tablets

Teva Presents New Long-Term AUSTEDO® (deutetrabenazine) Tablets Data at 2022 American Psychiatric Association ...